Supporting Information
The Imidazole-4,5-dicarboxylic Acid Scaffold
There is a growing need for bioactive chemical entities for use as tools in studying the cell and cellular processes, due in part to advances in high throughput screening protocols in combination with proteomic research that together are identifying new biological targets for possible therapeutic intervention in disease.
(Adang, 2001; al-Obeidi, 1998; Balkenhohl, 1996; Bleicher, 2003; Eliseev, 1999; Fenniri, 2000; Floyd, 1999; Golebiowski, 2001; Gorse, 1999; Gorse, 2000; Gray, 2001; Hann, 2001; Harper, 2004; Langer, 2003; Ma, 2001; Mason, 1999a; Nilakantan, 2002; Senderowitz, 2001; Thorpe, 2000; Weber, 2000; Young, 2004)*.
The research outlined in this proposal describes exciting new chemical entities based on an I45DA scaffold. The scaffold is structurally simple-yet readily diversified.
Moreover, the synthetic transformations to the various derivatives described in this plan are straightforward and few in number, thus enabling the synthesis of a greater amount of each target compound while retaining high analytical purity.
The chemistry to prepare these compounds allows the ready preparation of second-generation libraries or tagged analogs to also be uncomplicated. Examples of the structural classes and discovery compounds targeted in this proposal are shown on "Example Library Compounds".
In addition to these accessibility features significant for useful scaffolds, it is equally important that the I45DA derivatives yield conformations capable of representing known bioactive structures such as peptides
(Baures, 1999)*, protein surfaces (VanCompernolle, 2003)*, or nucleotides (see C.3.b). The compound classes in this proposal are largely unexamined in the literature, despite these aforementioned advantages
(Baures, 2005)*. The compounds with an I45DA scaffold that are known in the literature include those from international research groups and were prepared during the 70's and 80's
(Aleksandrova, 1976; Yasuda, 1983; Yasuda, 1987)*, models and intermediates for high-nitrogen content polymers
(Bouck, 1993)*, and a more recent class of bicyclic derivatives being examined for their potent bioactivities with viral drug targets
(Chen, 2000; Chen, 2001; Reayi, 2004a; Reayi, 2004b; Zhang, 2003a; Zhang, 2003b)*. My group has been developing the chemistry to yield related derivatives and new
classes of structures hypothesized to be bioactive
(Baures, 2002; Perchellet, 2005; Wiznycia, 2002; Wiznycia, 2004)*. Thus, very few of the many hundreds of compounds based on the I45DA scaffold have been studied, and nearly all are outside of existing patents.
Multiple research groups have described the strong intramolecular hydrogen bond found in I45DCs and I45EAs
(Aleksandrova, 1976; Bouck, 1993; Rush, 2005; Yasuda, 1987)*.
Our appreciation of the structural and conformational mimicry of I45DA-based derivatives with diverse natural structures like peptides and nucleotides was not foreseen when we began working with this scaffold as an alternate to traditional transition-state isosteres for aspartic protease inhibitors
(Baures, 1999)*. We have since, however, recognized that the hydrogen bond in I45DA-based derivatives is an adventitious feature for the scaffold.
Thus, our recent work has been to develop specific classes of I45DA derivatives for biological applications and to build the chemical expertise necessary to efficiently produce I45DA-based combinatorial libraries for discovery of bioactive small molecules (see C.4).
The I45DA-based compounds proposed herein generally fit the guidelines for drug-like compounds
(Lipinski, 1997; Lipinski, 2000; Muegge, 2001)*, including the number of pharmacophoric filter groups, a ring, fewer than five hydrogen bond donors and ten hydrogen bond acceptors, amphilicity, as well as molecular weights approximate to or lower than 500 g/mol.
These guidelines are neither a requirement for tools to be useful in studying cellular processes nor criteria for the pilot-scale libraries in this RFA. Rather, the I45DA derivatives simply have these features that could be important for future development.
In addition, we note that lead hopping from a bioactive I45DA-based compound to an alternative and already known drug-like scaffold is expected to be facile given our preliminary results (see C.3.b).
The aqueous solubility of I45DA derivatives has not been characterized in any detail to date. On the other hand, the least soluble of known I45DA derivatives are related to the symmetrically-N,N'-disubstituted I45DCs in specific aim 1, yet even these exceed the needed solubility limits in DMSO or CHCl3.
Indeed, a crystalline I45DC was used for 1H NMR spectroscopy at concentrations exceeding 1 M in DMSO-d6 and 100 mM in CDCl3
(Rush, 2005)*. All of these compounds will therefore meet the solubility guidelines in the RFA (10 mg in 2 mL of 90:10 CHCl3/MeOH-equivalent to 15 mM for the described model compound).
The bis-I45DCs reported to inhibit the binding of CD81 with the hepatitis C glycoprotein E2 used 6% DMSO in order to maintain their solubility at 4.8 mM in aqueous buffer
(VanCompernolle, 2003)*.
These compounds have molecular weights as large as any of the examples in the proposed libraries, are substituted with hydrophobic amino acids, and required micromolar concentrations to observe activity in the bioassay (best EC50 = 38 µM).
Thus, we consider this to be an example of a worst-case scenario, but one where the resulting compounds were still valuable research tools in the discovery of inhibitors of this protein-protein interaction on whole cells.
The other examples in our libraries are certain to have increased aqueous solubility, and we also reason that with the greater number of compounds proposed herein, the libraries could be tested at a concentration representing a greater threshold for activity, such as 10 µM.
We also recently reported on dissymmetrically-N,N'-disubstituted I45DCs with suitable aqueous solubilities for investigating their antiproliferative effects against HL-60 cells
(Perchellet, 2005)*.
The compounds my group has prepared to date (est. ~200 total) are largely solids, though frequently amorphous.
The exceptions have been the I45DCs with amino acids as both substituents on the ring, which instead yield viscous oils or glasses, perhaps due to multiple conformations present in solution.
On the other hand, all of the compounds prepared to date are chemically stable at room temperature, either in solution or in the solid-state.
Back to Background & Significance
Back to the Top
Intramolecular Hydrogen Bonding in I45DA Derivatives Key to Scaffold Utility
The strong intramolecular hydrogen bond in I45DCs or I45EAs predisposes these derivatives as a mimic of important biological structures on the basis of comparative distances between pharmacophoric groups (Figure 2). Hamilton, et al.*, has reported on the similar use of comparative distances coupled with scaffold conformational considerations in order to design substituted terphenyl α-helix mimics to antagonize biologically significant protein-protein interactions (Kutzki, 2002; Orner, 2001)*. These two dimensional comparisons are oversimplifications of the total conformations and dynamics sampled by these molecules (both the I45DCs and terphenyls). On the other hand, it is widely appreciated that preferred conformations of compounds restrict the relative topology of pharmacophoric groups and is a strong predictor of bioactivities. Such has been the premise of researchers designing peptidomimetics in order to mimic bioactive peptides (Hruby, 1993; Hruby, 1996; Hruby, 1997; Hruby, 2000; Marshall, 1993)*. This pharmacophore mimicry is also a feature of retro-inverso peptides, where the stereochemistry and sequence are both opposite that of a bioactive peptide (Fischer, 2003)*. Like these peptides, the substituents presented from the preferred intramolecular hydrogen bonded conformation of an I45DC compare favorably with side chain separations found in important protein secondary structures such as an α-helix or β-strand (Figure 2).
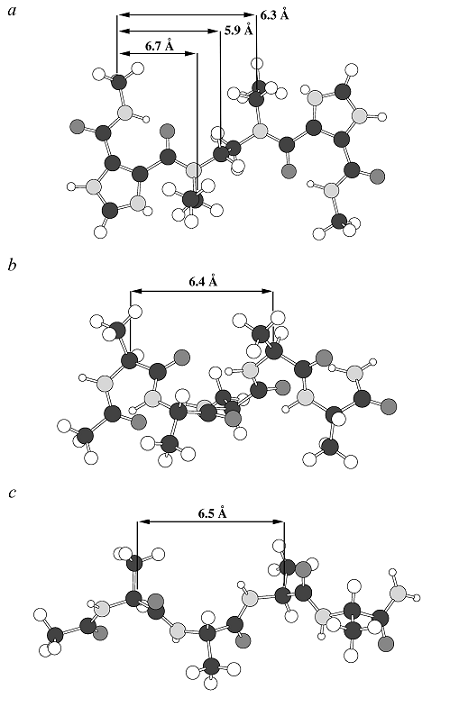
Figure 2. Comparison of distances between a) carbon atoms of an I45DC-based compound bearing substituents in one favored conformer with the α-carbons in an b) ideal α-helix and c) antiparallel β-strand.
It has been proposed that a compound library should sample a range of topographical space with a diversity of pharmacophoric substituents, as this provides the greatest opportunity for identifying a complementary interaction in the biomolecular recognition event
(Gorse, 2000)*. This certainly seems logical, yet there is also reason to expect compound libraries that use scaffolds that mimic important biological structures would be useful and informative as compared with data from solely a structurally diverse compound library
(al-Obeidi, 1999; Khersonsky, 2004; Kolb, 1998; Ma, 2001; Senderowitz, 2001)*. Such highly informative libraries could also significantly reduce the time, energy, and resources spent discovering lead structures against important biological targets.
Indeed, the bis-I45DC in Figure 2 was designed to mimic the surface of an α-helix found in tetraspanin CD81
(VanCompernolle, 2003)*. This functional I45DC-based mimic (best IC50 = 38 µM for <100 compounds tested) of a protein
illustrates the both validity and utility of this approach.
In both the I45DC and I45EA derivatives, the intramolecular hydrogen bonding interaction provides a shallow potential energy well-at least in comparison with covalent bonds-and thereby allows the scaffold greater conformational flexibility in adapting to its biological target (induced-fit model of binding).
Thus, the scaffold retains a potentially useful level of translational and rotational freedom about the intramolecular hydrogen bond (N-H...O) and substituent bonds that could be used in creating better contact between a compound and a macromolecular target.
We have learned by examining known literature examples
(Bouck, 1993; Yasuda, 1987)* and our own work
(Rush, 2005)* that the structure and conformation are intimately linked for this scaffold.
In contrast to many other scaffolds, relatively small changes in an I45DA derivative (e.g., a H-->CH3 substitution in one of multiple locations or an N-->O atom substitution within an amide to yield an ester) can produce significant conformational changes.
In this way, well-designed libraries of I45DA-based compounds provide valuable information about both binding interactions and conformation of the scaffold at the target.
Figure 2 emphasizes the distances between amino acid side chains in secondary structure with the distances between I45DC substituents, even though there are also hydrogen bond donor (N-H) and acceptor groups (C=O) between amino acid side chains in both the α-helix and the β-strand.
The hydrogen bond donors and acceptors within an α-helix sequence form intramolecular N-H...O hydrogen bonds that stabilize the structure of the α-helix. These hydrogen bonds are not expected to form significant contacts between a bioactive α-helix surface and a macromolecular target, since the atoms are sterically shielded from such contacts.
Thus, transferring the relative orientation and functionality of the side chains from a bioactive α-helix can yield bioactive I45DA-based α-helix mimics
(VanCompernolle, 2003)*
The β-strand hydrogen bond donors and acceptors, on the other hand, are expected to be involved in intermolecular contacts with macromolecular targets. A donor N-H and acceptor C=O between the i and i+2 amino acid side chains of a β-strand compare with the intramolecularly hydrogen bonded N-H and C=O of an I45DC.
Indeed, we have observed the formation of intermolecular N-H...O hydrogen bonds to the intramolecular hydrogen bonded donor and acceptor atoms in a sI45DC
(Baures, 2002)*.
Thus, on the basis of the observations detailed here and the data in our preliminary results, this scaffold is shown to be capable-given an appropriate set of substituents and context-of representing peptide, protein surface, or nucleotide structure.
Back to Background & Significance
Back to the Top
The Bioactivity and Toxicity of I45DA-Based Compounds
The usefulness of a compound as a probe of cellular processes requires that it have modest to potent bioactivity as well as specificity for a given target.
In addition, this must also be combined with acceptable (in light of the potency and specificity) physical properties such as solubility.
In the case of whole cell assays, a compound should also resist rapid degradation due to proteolysis or metabolism by oxidases.
The amide or ester bond adjacent to the imidazole ring is anticipated to resist rapid proteolysis due to steric constraints.
Ideally this should be a balance, as there is reason to expect the I45DA-based derivatives could also be valuable as active-site directed enzyme inhibitors.
It is noteworthy that ester hydrolysis more remote from the imidazole ring is apparent from our preliminary results, but is subject to the size of the ester group as well as nearby stereocenters
(Perchellet, 2005)*. The potential for phase I metabolic degradation by enzymes such as cytochrome P-450's does exist, although we have not performed any studies in this regard, nor are we aware of any others doing this work.
There are biologically active I45DA derivatives known in the literature.
It is unknown whether these compounds have selectivity for a single target or even for a single biochemical pathway, as most have never been widely tested in a manner to determine this.
Yet, it is reasonable to expect that suitable selectivity can be developed for compounds with bioactivity in the library classes, either in the primary I45DA-based libraries herein or in subsequent second-generation libraries.
The known bioactive I45DA-based compounds include glycarbylamide, the name given a structurally simple I45DC derivative that had been used at one time as an additive in chicken feed to prevent coccidiosis
(Cuckler, 1958)*. The FDA revoked the use of glycarbylamide in 1972
(Anonymous, 1972)*, though it has since been shown to be active against Cryptosporidium parvum
(McDonald, 1990)*. A structurally related antifein (a general name given this class of substances in the primary literature) has been shown to protect embryos from teratogens
(Bichevaya, 1999)*. Additional bioactivities reported in the literature for I45DC derivatives include their affect on memory
(Borodkin, 1984)* and their incorporation into antibiotics
(Yasuda, 1983)*. I45DCs also influence respiration by their similarity to the xanthines
(Bogoslavskaya, 1980)* and bind adenosine receptors
(Reikhardt, 1994)*. Their ability to mimic adenosine is particularly suggestive that these compounds could have bioactivities at other signal
transduction targets that bind purines, such as G-proteins, ion channels, or kinases. Some bicyclic derivatives, described as "fat" nucleoside analogues, have also recently been reported and have bioactivity against Flaviviridae NTPases/Helicases
(Chen, 2000; Chen, 2001; Zhang, 2003a; Zhang, 2003b)*. The bioactivity of I45DA derivatives generated by our laboratory is given in the preliminary results.
Back to Background & Significance
Back to the Top
Summary of Benefits for I45DA-Based Scaffolds
The I45DA-based scaffolds offer distinct advantages over most other known scaffolds in the discovery of new bioactive lead structures.
The I45DA derivatives are easy to synthesize and are diversified by both functional group incorporation as well as by changing the derivative class.
The relatively small structural changes between some derivative classes can have large conformational implications, providing valuable information for developing the structure-activity relationship (SAR) for a bioactive derivative.
The scaffold has a strong intramolecular hydrogen bond so as to predispose the substituents in relative space, while offering adaptability of the derivative to a binding site.
In addition, transformation of the SAR from these compounds into an alternative and drug-like purine ring system is expected to be facile, thereby providing a method for altering the physical properties of a lead compound without having to rediscover the activity in a new scaffold.
Back to Background & Significance
Back to the Top
Synthesis of I45DA Derivatives
General Information
To date we have synthesized approximately 100 I45DCs with varied substituents and nearly an equivalent number of oligomeric I45DC-based analogs. We have also synthesized a small number (<10) of I45EAs and I45DEs derivatives. All of these compounds have been characterized by using 1H and 13C NMR spectroscopy, mass spectrometry, and in the case of many monomeric representatives, by elemental analysis.
Symmetric N,N'-Disubstituted Imidazole-4,5-dicarboxamides (sI45DC)
These compounds are the simplest derivatives to prepare, and we have reported on the synthesis of representative examples (Baures, 1999; Baures, 2002; VanCompernolle, 2003; Wiznycia, 2004)*. The synthetic methodology begins with the formation of the reactive pyrazine, 1.2, from I45DA as shown in Scheme 1. Addition of alkanamines to this intermediate results in the formation of 1.3. Yields are generally high (> 80%), although the reactivity of 1.2 prevents purification of this intermediate. Thus, selective solubilization, solid-phase extraction, or crystallization is generally used to purify the subsequent reaction mixture. Of these three purification methods, the passage of the reaction mixture through a pad of silica gel works the reliably across the diversity of structures within this series, and separates the desired I45DC product from the hydrolyzed byproducts that are the significant impurities (monosubstituted and I45DA).
Dissymmetric N,N'-Disubstituted Imidazole-4,5-dicarboxamides (dI45DC)
I45DC Substituted with Alkanamines
The reactivity of both the acid chloride and acyl imidazole functionalities have prevented us from controlling the stoichiometric and subsequent addition of two different amines in order to yield the dI45DCs. Our attempts to prepare dI45DCs in this manner have all yielded mixtures of the desired product along with the two sI45DC possibilities. Our solution to this problem was to modulate the reactivity of the acid chloride by first forming the pyrazine ester 2.1 with phenol, as shown in Scheme 2 (Wiznycia, 2002)*. The acyl imidazole bond could then be opened with one amine to give 2.2, and the phenol ester converted to an amide by addition of a second amine to give 2.3. This route more than doubled the isolated yield to these derivatives over the literature method, and it also reduced the total synthesis by one step.
I45DC Substituted with Amino Acids
Although alkanamines are too reactive to offer complete selectivity between the acid chloride and acyl imidazole, amino acid esters do react with selectivity. Thus, the addition of two equivalents of amino acid ester in the presence of a base yields the substituted pyrazines 3.1 as shown in Scheme 3. These intermediates can be crystallized from CH2Cl2/hexanes to give products of high purity (< 95% as evident by 1H NMR spectroscopy) for the subsequent reaction. Addition of amines to 3.1 yields 3.2, and the addition of diamines to 3.1 is the method by which the bis-I45DCs, 3.3, were prepared (Wiznycia, 2004)*.
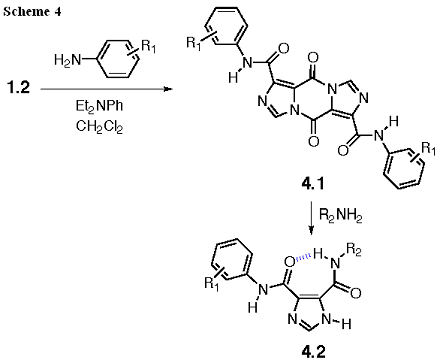
I45DC Substituted with Anilines
Anilines, in the presence of diethylaniline as an acid scavenger, also react selectively with the acid chloride in 1.2, thereby providing 4.1 as shown in Scheme 4. The remaining acyl imidazole bonds can be reacted with amines (including amino acid esters) in order to yield 4.2. We have prepared approximately 40 of these analogs in the past year and had them screened, in collaboration, for their antiproliferative effects against HL-60 cells (Perchellet, 2005)*.
Imidazole-4-Ester-5-Carboxamides (I45EA)
The I45EAs are readily prepared by reacting 1.2 with an alcohol at room temperature, followed by the opening of the acyl imidazole bond with a reactive amine. The intermediate pyrazine ester can be obtained in sufficient purity (> 90%) by filtration if no base is used (Baures, 2002)*. In the presence of a base as an acid scavenger, the entire reaction can be done in a single pot.
Imidazole-4,5-Diesters (I45DE)
We have not had reason to prepare many I45DEs for the projects undertaken thus far, though we did recently report the synthesis of one compound in this class (Rush, 2005)*.
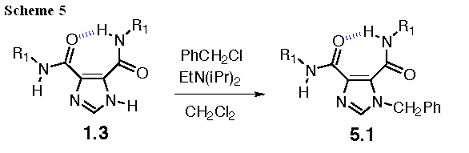
Ring-Alkylated I45DC
The imidazole ring is a relatively poor base (and nucleophile) in the I45DCs. Nonetheless, alkylation of 1.3 with benzyl chloride yields 5.1 in near quantitative yield (Scheme 5). This is the only example of this class of derivatives that we have made to date.
Bis-I45DC
The synthesis of these derivatives follows that shown in Scheme 3, as recently reported (Wiznycia, 2004)*, by the addition of different diamines to pyrazines 3.1.
Back to Preliminary Work
Back to the Top
Conformational Analysis and Physical Properties of I45DA Derivatives
The I45DCs and I45EAs have strong intramolecular hydrogen bonds that have been described by others (Aleksandrova, 1976; Bouck, 1993; Yasuda, 1987)*. We have invested a considerable amount of time determining the strength of this interaction in a representative I45DC. As shown in Figure 3, the benzylamine disubstituted I45DC has two distinct amide NH signals when recorded at 5 mM in DMSO-d6. Also shown in this figure is the chemical shift of the imidazole NH that is both solvent and
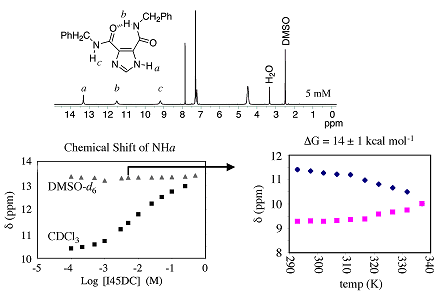
Figure 3. 1H NMR data that highlights the two distinct amide NHs (top), the imidazole NH chemical shift as a function of solvent and concentration (lower left), and the influence of temperature on the two amide NH chemical shifts of this I45DC when recorded at 3 mM in DMSO-d6.
concentration dependent, as these compounds form hydrogen bonded aggregates at higher concentrations in non-polar solvents like CDCl3. A variable temperature 1H NMR spectroscopy study of the amide NHs in the benzylamine disubstituted I45DC at 3 mM in DMSO-d6 (lower right graph in Figure 3) puts a lower limit on the intramolecular hydrogen bond at 14±1 kcal/mol (Rush, 2005)*. We have also shown that the intramolecular hydrogen bond is stable in water at neutral or slightly acidic pH, as shown in Figure 4. An increase of acidity results in protonation of the imidazole and disrupts the hydrogen bond. The dynamics of this process begins to occur on the NMR time scale around pH 5, although the pKa of the imidazolium ring is less than 2 (Rush, 2005)*.
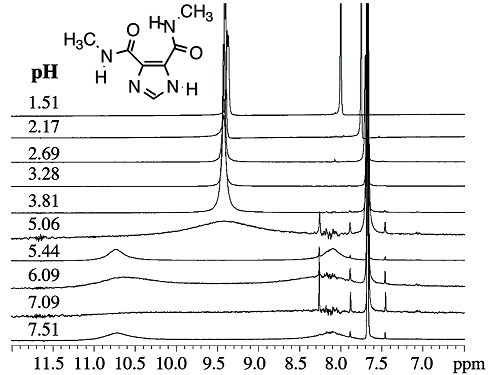
Figure 4. 1H NMR data for the shown I45DC, recorded in 30% DMSO-d6/H2O by using a water suppression program. The intramolecular hydrogen bond is still present at neutral pH, but is more dynamic below pH 5.
Back to Preliminary Work
Back to the Top
Bioactivity of I45DA Derivatives
The bioactivity of I45DCs and I45EAs reported by other groups was highlighted earlier (see The Bioactivity and Toxicity of I45DA-Based Compounds). We have used the I45DC scaffold in several projects. The design and resulting bioactivity of the compounds employed for these applications is detailed in the following sections.
Heterocyclic HIV-1 Protease Inhibitors
Our first use of the I45DC scaffold was in the design of heterocyclic HIV-1 protease inhibitors (Baures, 1999)*. The best I45DC-based inhibitor, out of less than 20 compounds tested to date, has an IC50 value of 0.3 µM. Part of the design strategy and the hypothesized active site interactions are shown in Figure 5. We do not expect the I45DC to retain the intramolecular hydrogen bond when bound, but to be in an extended conformation instead. This is supported by the fact that the intramolecular hydrogen bond exchanges rapidly on the NMR time scale below pH 5 (Rush, 2005)* and that the bioassay was done in acetate buffer at pH 4.7.
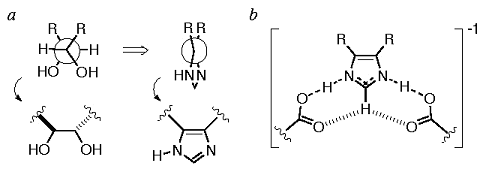
Figure 5. a) The design of the imidazole ring was based on the conformation of a dihydroxyethylene inhibitor bound to HIV-1 protease, wherein the two alcohols are gauche. b) The hypothesized interaction of the I45DC with the aspartic acids of the catalytic active site. The diagram indicates contributors resulting from symmetry-equivalent hydrogen bonded structures, plus interactions resulting from proton transfer by an aspartic acid side chain (partial bonds). The potential of the ring C2-H to form an interaction is indicated by the dashes. and that the bioassay was done in acetate buffer at pH 4.7.
Phosphodiesterase-4 (PDE-4) Inhibitors
The formation and stability of the intramolecular hydrogen bond at neutral pH in water led us to consider the interaction as a quasi ring, and in this way, the structure resembles that of a substituted purine. Selected I45DCs were tested at 100 µM against PDE-4 by contract (MDS Panlabs). The benzylamine sI45DC shown in Figure 6 inhibited PDE-4 by 50%, whereas the reported IC50 for a comparable adenosine is 29 ± 6 µM against PDE-4 and greater than 200 µM against PDE-3 (Crespo, 1998)*.
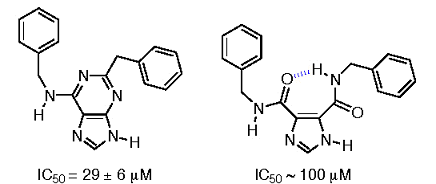
Figure 6. Comparison of PDE-4 inhibition by a substituted adenosine and structurally similar I45DC.
Protein-Protein Interaction Inhibitors
The X-ray crystallography data on the I45DCs (Baures, 2002)* provided the inspiration for generating protein-protein inhibitors with the I45DC scaffold. We recognized the similarity in distance between the two substituents on an I45DC as compared with the Cα-Cα distance (6.4 Å) between adjacent side chains on the same face of an ideal α-helix (Figure 7). The Cα-Cα distance between adjacent side chains (3.8 Å) along an ideal α-helix is also shown in Figure 7.
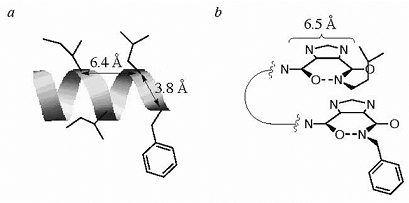
Figure 7. a) Distances between select hydrophobic side chains in the CD81 helix D as determined from an ideal α-helix backbone. b) A possible mimic of this α-helix created by the connection of two imidazole rings through a linker either containing two additional amino acid side chains or without these additional side chains.
Human CD81 (hCD81) has been proposed to be a receptor (or coreceptor) used for cellular entry by the hepatitis C virus (Pileri, 1998)*, and several labs have examined the binding interaction between human CD81 and the hepatitis C glycoprotein-E2 (HCV-E2) (Drummer, 2002; Hadlock, 2000; Higginbottom, 2000; Petracca, 2002; Wunschmann, 2000)*. Two X-ray crystal structures of the large extracellular loop (LEL) of hCD81 provided valuable information regarding the structure of the loops in the hypervariable region (HVR) (Kitadokoro, 2001; Kitadokoro, 2002)*. The LEL contains several α-helices, including helix C and helix D that are formed by the amino acids between two disulfide bridges. These sequences served as the basis for our design of a series of bis-I45DCs that were synthesized and tested for their ability to inhibit the binding interaction between recombinant HCV-E2 and Molt-4 T cells expressing CD81 (VanCompernolle, 2003)*. The best bis-I45DC tested to date has an IC50 = 38 µM in this bioassay. We recently reported the synthetic routes and analytical data for the bis-I45DCs (Wiznycia, 2004)*.
Antiproliferative I45DCS: Potential Kinase Inhibitors
Protein kinases form the largest protein family in the human genome and have an important role in signal transduction within the cell
(Sielecki, 2000)*. Moreover, protein kinases are at the center of events that help control the cell cycle and as such are valued targets for cancer chemotherapy
(Dancey, 2003; Grant, 2003; Haesslein, 2002; Malumbres, 2001; Mareel, 2003; Sausville, 2003; Scapin, 2002; Sielecki, 2000)*.
Many classes of compounds interfere with kinase activity by binding competitively to the ATP binding site
(Cherry, 2004; García-Echeverría, 2000; Gray, 1999; Haesslein, 2002; Li, 2004; Noble, 2004; Scapin, 2002; Sielecki, 2000; Toledo, 1999; Traxler, 1999)*.
X-ray crystallography of kinase complexes with known inhibitors across structural classes finds that pair-wise hydrogen bonding between the inhibitor and backbone N-H and C=O functionalities is a common feature in each class, as shown in Figure 8
(Cherry, 2004; Gray, 1999; Noble, 2004; Scapin, 2002; Sielecki, 2000; Toledo, 1999; Traxler, 1999)*.
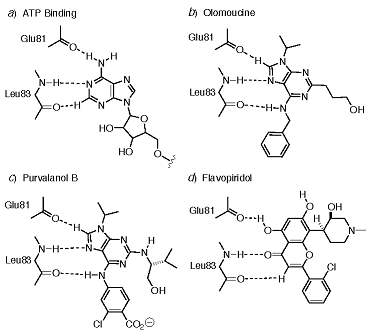
Figure 8. Comparison of ATP-competitive binding modes for different inhibitors of kinases. In all cases, pair-wise hydrogen bonds are formed with Leu83 of the kinase. Inhibitor hydrogen bonding also involves Glu81 (residue numbering as for cyclin-dependent kinase 2, cdk2).
These hydrogen bonds have been prominently featured in structure-based drug design projects against different kinase targets
(Goldberg, 2003; Honma, 2001)*.
Our design of potential kinase inhibitors begins with the intermolecular pair-wise hydrogen bonding observed in the solid-state for I45DCs and I45EAs
(Baures, 2002)* and uses these interactions as an "anchor" within the kinase ATP binding site as shown in Figure 9.
We also used the X-ray crystal
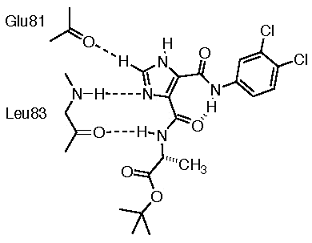
Figure 9. An I45DC in a hypothesized binding interaction with ATP-binding site residues found in cdk2. This compound is cytotoxic to HL-60 cells at 4 days with an IC50 = 2.5 ± 0.2 µM (unpublished).
structures of inhibitor bound cyclin dependent kinase 2 (cdk2) in order to hypothesize about the structure of the R-groups that could probe the edges of the ATP binding site for favorable and specific kinase interactions
(Li, 2003; Naumann, 2002)*.
It was anticipated that the intramolecular hydrogen bond could provide conformational flexibility within the kinase active site that may be important for the induced-fit of the inhibitor as well as for controlling the kinase activity that is linked to conformational changes of the protein
(Engh, 2002)*.
In selecting the starting I45DC, we chose a 3,4-dichloroaniline substituent, rather the 3-chloro-4-carboxyaniline present in purvalanol B.
This substitution seemed reasonable since the absence of the carboxy group yields purvalanol A, a compound that is also a kinase inhibitor.
In addition, purvalanol B is inactive on cells, possibly due to an inability to cross cell membranes with the presence of a charged functional group.
The second I45DC substituent was then varied in order to obtain a structure-activity relationship with respect to this starting structure.
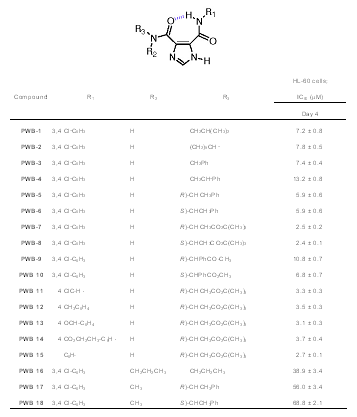
For our preliminary results, the antiproliferative activity of a series of I45DCs against HL-60 cells was used as a surrogate measure of their possible kinase inhibition.
This data, a portion of which is shown in Table 1, was determined in the laboratory of J. P. Perchellet at Kansas State University and was recently reported
(Perchellet, 2005)*.
The validity of this comparison is supported by recent results for the cyclin-dependent kinase inhibitor known as MCS-C2, where its cdc2 inhibition correlates with the viability of HL-60 cells
(Kim, 2003)*.
The most antiproliferative compounds in the series at 4 days have an IC50 ≈ 2.5 µM (PWB-7, PWB-8).
Combined, the structure-activity relationship of the compounds is consistent with a binding mode for cdk2 kinase, and provides a lead structure for further development.
The work also illustrates the appropriate solubility and stability of dI45DCs to serve as biological probes of living systems.
Back to Preliminary Work
Back to the Top
Oligomer Library Synthesis and Purification: A Model Scale-Up
To illustrate the ability to provide increased numbers of I45DA derivatives in a timely fashion, a small library of bis-I45DCs were synthesized, purified, and characterized over a 10-day period by the PI while still meeting all other responsibilities. As shown in Figure 10, a glass 96-well plate was used to contain the reactions. The reactions containing N,N'-dialkylalkanamines (rows D-G) gave intensely colored side products in addition to the desired compounds. Each reaction was analyzed by thin layer chromatography (TLC) before loading the concentrated reaction mixture onto homemade silica gel columns and eluting with a solvent gradient. While only 42 products were obtained pure after the first pass through silica gel columns, many of the remaining products were substantially purified (est > 90% clean) from this step. Product amounts for purified reactions ranged from 5-15 mg. These amounts represent poorer yields (~20-60 %) compared to individual reactions, but we expect that we can modify this experiment based on our result, and thereby greatly improve both the yields and the number of purified compounds obtained from such an experiment. It is noteworthy that the bis-I45DCs represent the most challenging of the compounds to purify to date, and most of the compounds proposed herein will be easily purified by solid-phase extraction on silica gel.
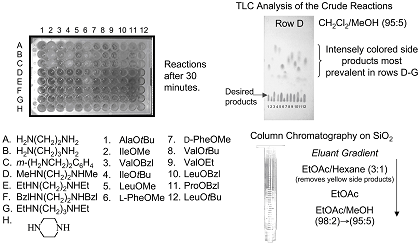
Figure 10. The reactions (upper left) of a small library prepared by parallel synthesis. The TLC of the crude reactions from row D is shown, as is an example of the homemade silica gel columns used to purify the reactions.
Back to Preliminary Work
Back to the Top